WHO PQ – What, How and WHY ?
World Health Organisation Prequalification (WHO PQ) programme helps countries having low and medium levelled incomes, by providing them with healthcare products with better safety, quality and efficacy. The healthcare products include in-vitro diagnostics (IVDs), medicines, vaccines, immunization devices, male circumcision devices and Cold chain equipment. The programme ensures that the above-mentioned key products meet the global regulatory standards. By getting the healthcare products qualified under this programme allows it to be used by UN Countries and other procurement agencies for purchasing for its further use. WHO publishes the list of prequalified products on its website as and when the data on such products has been assessed and evaluated by the WHO. The programme has enabled a huge market (approximately of USD 3.5 Billion) of quality, safe and effective healthcareproducts that are likely procured by national governments as well as private sector organizations.
WHO PQ for IVD Procedure overview
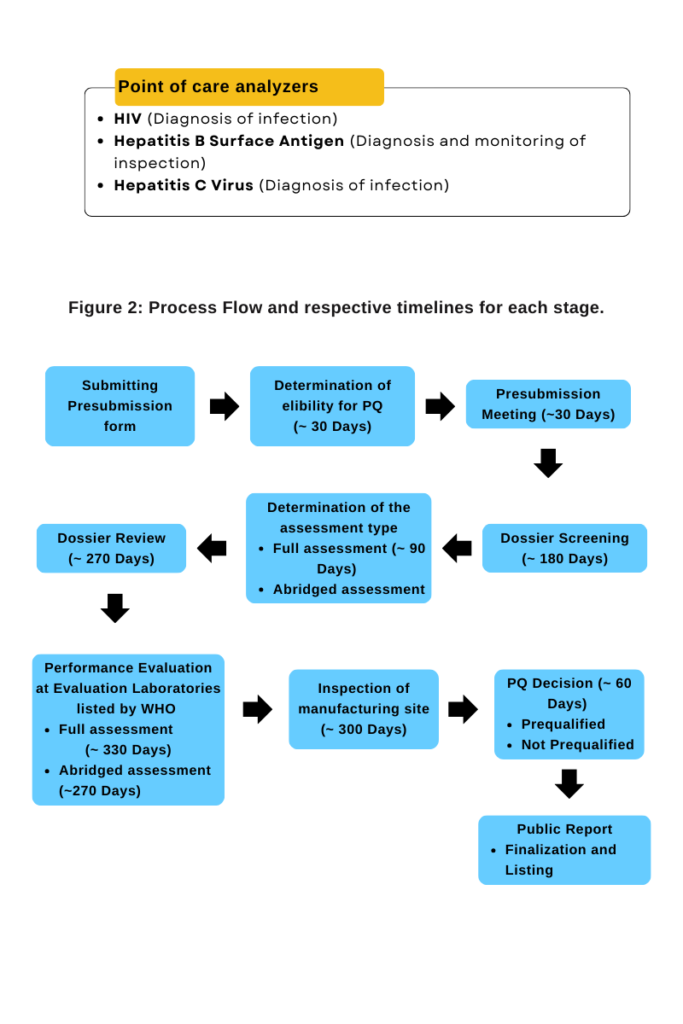
A wide range of diagnostics for endemic and epidemic diseases are covered by WHO PQ for IVD products. The programme assesses if product meets the PQ requirements based on three components, i.e. Product Dossier review, Laboratory evaluation of performance and operational characteristics and Manufacturing Site inspection. The aim of the performance evaluation is to independently verify the manufacturer’s claim regarding product performance. In addition, if UN procurement criteria have been established, performance evaluation enables it to be determined whether these criteria have been met.


Low and medium incomed countries are in a frequent demand of prequalified products for the prevention, diagnosis and treatment of diseases. It is also important for manufacturers who wish to get their products prequalified under this programme, to understand that getting the products qualified under this
programme doesn’t mean that they are endorsed or approved by WHO for its use, i.e. it is not a type of a regulatory approval that can be used by manufacturers as a proof for further referencing. It can be arduous for small manufacturers to meet the programme’s requirements. WHO offers technical assistance to IVD manufacturers to help them understand and comply with the PQ requirements and identify any major drawbacks or lapses in attaining compliance with international regulatory requirements and standards. The programme also helps manufacturers to build their capacity to produce.
Manufacturers should consider following aspects before planning to apply for WHO PQ as these points might help them expedite the process for review and the complete assessment process –
- The product development should be complete, i.e. all the performance parameters and the relevant product claims should be finalized before applying for WHO PQ.
- All the technical documentation shall be in line with the requirements stated out in the product-specific Technical Specification Series (TSS) and Technical Guidance Series (TGS) published by WHO.
- The QMS system of the manufacturer should be strong and compliant to ISO 13485:2016.
- The manufacturer should know the fees and timelines of the whole approval process and plan accordingly the application.
- The manufacturer should know along with the application and approval process, what they are supposed to do after getting the approval in order to maintain the WHO Prequalification status of their product.
Alceon Medtech Consulting is here to help you know your organization’s readiness for applying for WHO Prequalification of your product and meet all its requirements. Reach us to evaluate existing status of your system with respect to WHO PQ requirements and allow us to map the right directions for you to get your products WHO Prequalified. Inquire today at contact@alceonconsulting.com.
