What is a Design History File (DHF)?
The design history file is a compilation of records that describes the design history of the finished device.
The main purpose of DHF is to outline the design and development process of the medical device from start to end and ensure that the design fulfils the regulatory requirements.
The main purpose of DHF is to outline the design and development process of the medical device from start to end and ensure that the design fulfils the regulatory requirements.
To demonstrate that the device performs as intended and the appropriate requirements for the medical device have been met.
The design history file either contains or references the necessary documents from the DMR. It means that you could either create a folder that contains documents or create a document that acts as a reference sheet for the required materials.

Each manufacturer needs to establish and maintain a design history file (DHF) for each type or family of medical device.
What needs to be covered in your Design History File?
The critical elements that need to be covered in the design history file include the design and development plan, design input, design output, risk analysis, design review, design verification, design validation, design transfer, and design changes.
Below waterfall diagram represents all stages of the design and development
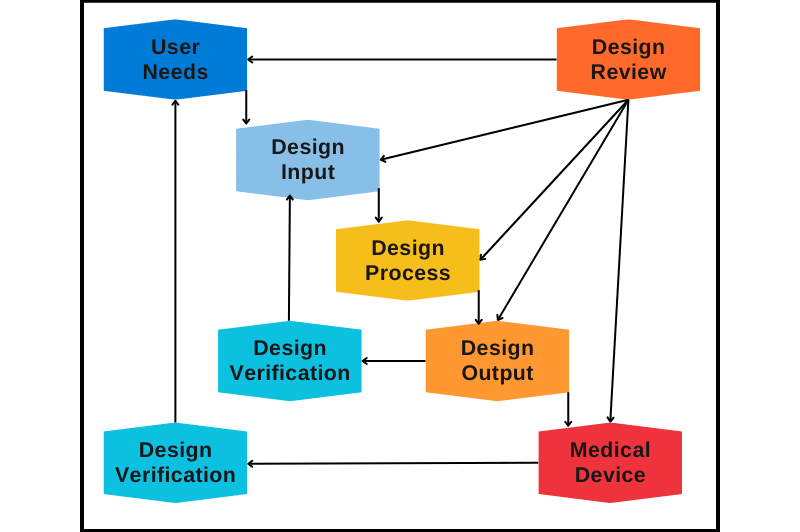
Figure 1: Stages of Design and Development Activities
Here we discuss the details of the design and development activities:
1. Design Plan
A design plan is essentially a road map (or recipe) for the development project. Each manufacturer needs to establish and maintain plans to ensure appropriate design and development activities, with defined responsibilities, identified review stage, identification of internal and external resources, and milestones and timelines for each activity should be planned. The plan must be reviewed, updated, and approved as design and development evolve.
Design plan may not be required for those design history files prepared retrospectively.

2. User Needs
The initial stage in the design and development of the device is to identify and understand the requirements of different stakeholders involved in healthcare. Common user requirements for the device that must be taken into account include safety and reliability, ease of use, compatibility with other devices, durability and maintenance, portability and accessibility, cost-effectiveness, data management customisation and flexibility, and regulatory compliance. Input on these needs can be collected through interviews, surveys, observations, and testing.
Here we discuss the details of the design and development activities:
3. Design Input
Design input is the most critical element of the design control process.
Design inputs are “the physical and performance requirements of a device used as a basis for device design.” They are the starting points and provide the foundation for successful product design and development. Inputs must be comprehensive, realistic, and defined in unambiguous and quantifiable terms.
Design inputs typically cover four areas:
1. clinical use, safety, and performance
2. product physical and performance characteristics,
3. marketing requirements,
4. regulatory and/or quality requirements.
Sources for the collection of these inputs can include the following: scientific literature, previous similar designs, market surveys and research, clinical practices, risk hazard analysis, predictive or similar devices, product-specific standards.

Here are some examples of design input for a hypodermic syringe:

Figure 2: List Design Input for Syringe
4. Design Output
Design outputs are “the result of an effort at each design phase and the end of the total design effort.” The finished design output is the basis for the device master record (DMR). The total finished design output consists of the specifications for the device, drawings, its packaging and labelling, and the DMR.
Here are some examples of design outputs:
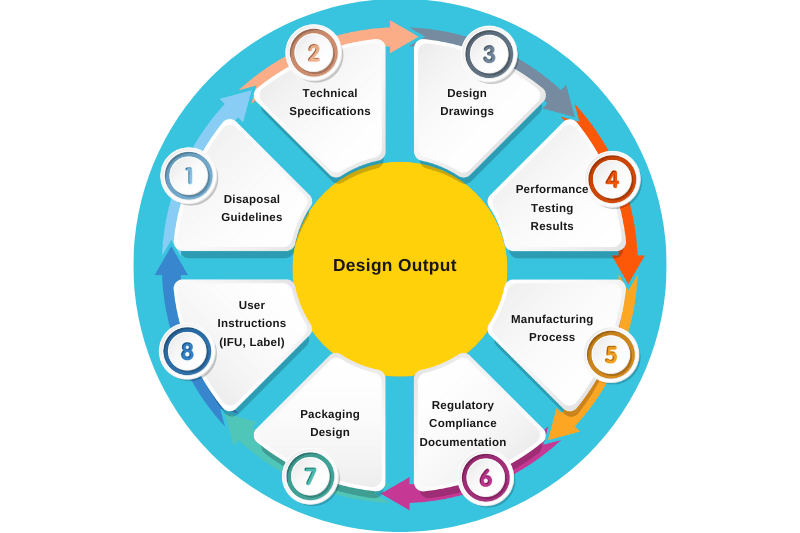
Figure 3: List of Design Outputs of Syringe
5. Design Verification
Once the device design has been finalised, design verification can take place to provide objective evidence through measurable means that the design outputs from the final device design meet the design input requirements. Device design can be verified using several verification methods, including product testing, record review, comparing the new design to the previous design of a similar product, and analysis or measurements. Objective evidence shall include plans, specifications, test reports, and other records like labels and IFU.

6. Design validation
Once the design has been verified, design validation must be carried out to confirm that the final product design meets the user needs. This is usually performed through a clinical evaluation with actual or simulated users and utilises representative products to ensure that the results of the studies reflect real-world expectations.

7. Design Review
Design review shall be performed at appropriate stages to evaluate the ability of the results of the design to meet the requirements and identify and propose the necessary actions. Stages at which reviews are conducted usually are after identifying the inputs, when the outputs are finalised, at different stages of design verification, and so on.
8. Design Transfer
Design transfer is the process of transitioning design from the development stage to production. This step ensures that the device can be produced consistently and meets all specifications and regulatory requirements. Design transfer checklist needs to be prepared and maintained to ensure that all required documents are ready for transfer to production.
9. Design Changes
A product’s design can change after it is released in the market. Such design changes must be documented.
Manufacturers need to establish and maintain procedures for the identification, documentation, validation, or where appropriate, verification, review, and approval of the design changes before their implementation.

The design changes document must specify the changes.
An example is provided as follows:
- User interface improvements
- Device ergonomic feature
- The intended use, indication, contraindication, warnings
- Performance characteristics
- Supplier
- The intended user groups
- Material from which the device made
- Characteristics not yet considered by the clinical evaluation
- As a result of considerations arising from market surveillance, including incidents, recalls, or complaints.
Design changes need a thorough risk assessment prior to the change to be certain that the changes do not impact the performance or safety of the product. Major design changes may also need notification to Regulatory authorities.
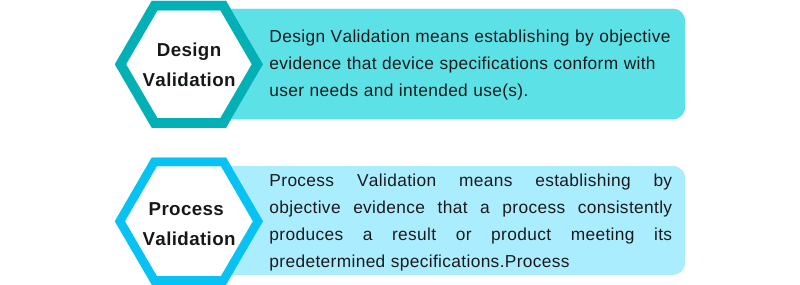
Confused with Design Validation and Process Validation
We have often found that there is confusion around design and process validation. Let’s understand that design validation is not the same as process validation. Process validation is usually a part of the design transfer activities.