In the ever-changing landscape of medical device regulation, technology plays a vital role in improving efficiency and ensuring robust processes. The eSTAR program launched by the US Food and Drug Administration (FDA) is a great example of leveraging technology to transform regulatory processes. All 510(k) submissions must be made electronically via eSTAR from October 1, 2023.
This blog will explore the eSTAR program and its significant impact on FDA operations.
What is eSTAR?
The eSTAR program, which stands for Electronic Submission, Template, and Resources, was initiated by the FDA to modernize and streamline the regulatory review and approval process for medical devices. The goal is to replace traditional paper-based filing and manual tracking systems with a comprehensive electronic platform.
The eSTAR templates are interactive PDF forms designed to guide users in creating comprehensive 510k submissions for medical devices except for exempted devices and pre-market approval. These submissions can be for traditional, special, or abbreviated 510k applications and de Novo applications.
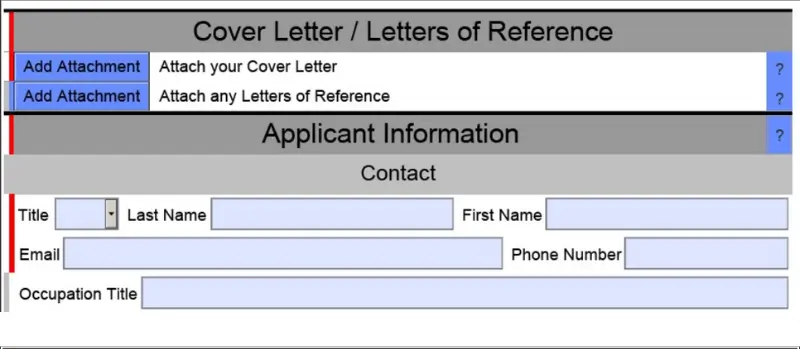
Providing some background information, the eSTAR template is a structured form that systematically takes users through each section and question of the 510k submission. The questions are specifically designed to help users determine the necessary information that needs to be included in their 510k submissions.
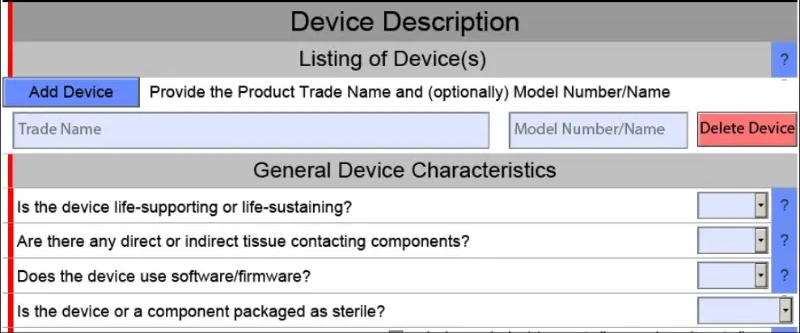
Availability
- eSTAR templates are free.
- There are two separate forms available, one for non-IVD devices and another for IVD devices.
- Interestingly, both 510k and De Novo submissions can be created using the same forms.
- Another form is available for early submission requests, i.e., PreSTAR, Pre-Submissions (a type of Q-Submission) for non-in vitro and In Vitro Diagnostic devices.
- eSTAR is voluntary for medical device De Novo submissions to CDRH or CBER.
- eSTAR is also voluntary for medical device pre-submissions to CDRH.
- The eSTAR is not currently for use with combination products.

Difference with the current process
Both the eSubmitter and eSTAR programs are electronic submission tools developed by the FDA to help medical device manufacturers submit their approval documents more efficiently. The eSTAR program comes after the eSubmitter initiative as the next step in the digitalisation process.
| eSTAR | eSubmitter |
|---|---|
| It is an interactive PDF form that guides applicants through the submission process. The PDF enables attachments to be included. | It enables the creation of supplements and amendments. |
| The template uses Adobe Acrobat Pro or DC rather than a proprietary program. | Improved Data Integrity: With eSTAR, the FDA receives standardised, structured, and electronically verifiable data. |
| This minimises the potential for errors and inconsistencies, ensuring high-quality data for regulatory decision-making. | With the eSubmitter program, there is no default structured data or document structure like the eSTAR program. |
| It enables the creation of supplements and Amendments. | This require There was no automation in the software for an RTA assessment. |
| Eliminates the need for an eCopy validation and RTA assessment. | It enables the creation of supplements and amendments. |
| This require There was no automation in the software.d an RTA assessment. | Improved Data Integrity: With eSTAR, the FDA receives standardised, structured, and electronically verifiable data. |
| Automated confirmation and verification. | There was no automation in the software. |


Although it may appear to be a helpful and straightforward process, executing it is not as effortless as it might initially seem.
- In some sections, the template includes a field that can be used to enter the requested information or explain why that information is not needed or why an alternative method is used. In other parts of the form, attachments will be required to include this type of information.
- Text boxes also cannot contain tables or figures, which are often useful, especially for comparing a predictive device and/or explaining the technological characteristics of a device.
Review Timelines:
- A technical screening will be completed in 15 calendar days instead of conducting an RTA screening.
- If the eSTAR does not pass technical screening, FDA will notify the submitter via email and identify the incomplete information, and the 510(k) will be placed and remain on hold until a complete replacement eSTAR is submitted to FDA.
- If a replacement eSTAR is not received within 180 days of the date of technical screening deficiency notification, FDA will consider the 510(k) to be withdrawn and the submission will be closed in the system.
- The 180-day deadline for responding to an additional information (AI) request has not changed in the eSTAR draft guidance.

Reach out to us to assess your current regulatory approach and let us chart the most effective course for you. Whether you need to upskill your team or streamline your documentation, we’ve got you covered. Inquire today at contact@alceonconsulting.com

