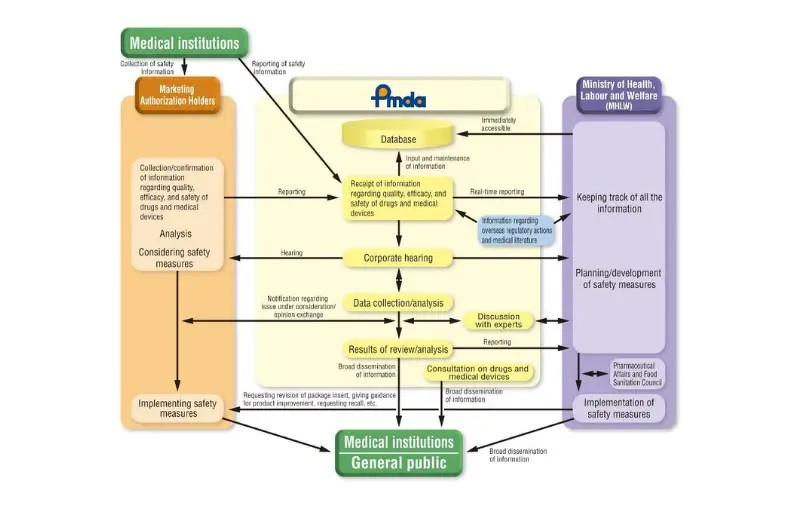
The Pharmaceutical and Medical Device Act (PMD Act) governs medical devices in Japan, which sets the requirements for pre-market approval and post-market Surveillance activities. It is enforced by PMDA(Pharmaceuticals and Medical Devices Agency) and MHLW(Ministry of Health, Labour and Welfare).
Reporting Timeframe:
Adverse events related to serious injuries or deaths must be reported to PMDA within 10 days.
Database:
Japan maintains a database for adverse event reporting, which PMDA monitors. Healthcare professionals and manufacturers are responsible for reporting adverse events. Reports are typically submitted via the PMDA’s Adverse Event Reporting System through electronic channels such as the Medical Device Safety Information System or by paper forms, ensuring that the data is collected, monitored, and analyzed for safety actions.
The PMDA provides the following safety information regarding medical devices.
1.PMDA Risk Communications
(medical devices risk information of ongoing evaluation)

2.Revisions of PRECAUTIONS
contain the information issued by the MHLW regarding medical devices on revisions of PRECAUTIONS in package inserts.
3.MHLW Pharmaceuticals and Medical Devices Safety Information (PMDSI)
is intended to facilitate the safer use of drugs and medical devices by healthcare professionals.
4.Notification on Self-inspection
contains notifications on the self-inspection of medical devices issued by the MHLW.
4.Notification on Self-inspection
contains notifications on the self-inspection of medical devices issued by the MHLW.
5.PMDA Alert for Proper Use of Medical Devices
6.PMDA Alert for Proper Use of Medical Devices (for patients)
7.Notifications Related to Safety Measures (medical devices)
PMDA Website: https://www.pmda.go.jp
MHLW Website: https://www.mhlw.go.jp