All right, we’re back with more content on reprocessing of reusable medical devices. We introduced this topic in our last blog, where we discussed the fundamentals of reprocessing, types of reusable medical devices, the three broad levels of the process and the role of the manufacturer. This blog will discuss the nitty-gritty of selecting a worst-case while planning for re-processing validation. Let us start by elaborating on what the term ‘worst-case’ means. How about an example? Assume that you’re a manufacturer of surgical instruments, manufacturing the following products:
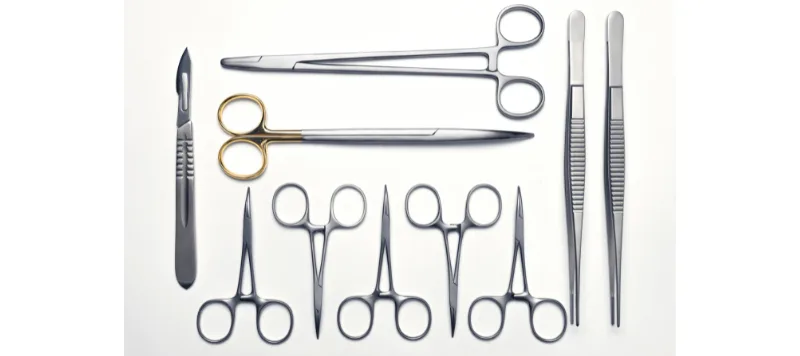
Image 1: Surgical instruments (mix of scissors, holders, forceps)
Since these instruments are reusable in nature, you’d agree that these need to be reprocessed (i.e. cleaned, disinfected and/or sterilized) before each subsequent use. To claim this, you, as the manufacturer, need to validate the reprocessing instructions.
Now, the question is, will you include all devices in the validation? Well, you can. But think about the enormous amount of time, resources and cost involved. This is precisely where selecting worst-case(s) pays off!
Technically, worst-case acts as a representative device(s) that poses the highest challenges or is most difficult to meet the acceptance criteria. So, if the worst case meets the acceptance criteria, one could assume that the other devices in the group would also meet the acceptance criteria.
One of the common questions we come across is, does the worst-case mean only one device? Well, indeed no. There may be more than one device that could qualify as a worst case, and you may need to include multiple devices during the validation; however, it all depends on the intended use of the devices and the product scope selected for validation.
So, how do you identify these worst cases? The simple logic says that the device that is most difficult to clean in actual practice. That does not sound like much work, doesn’t it? Keep going..!
To help identify ‘the-most-difficult-to-clean-device(s)’, the following evaluation parameters may be used:
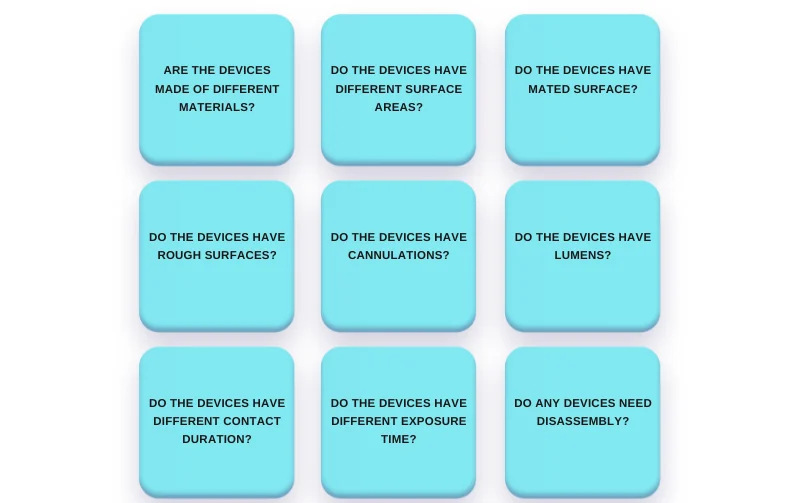
Image 2: Evaluation parameters for worst-case identification
The above-mentioned parameters do not make a definite list for all types of devices. There’s more to them, but it depends on devices’ intended applications and design complexities.
Honestly, identifying worst cases while planning for validation is a complex and time-taking task. Considering the design, intended applications as well as real-world usage of the devices help get to the final decision. Sounds quite a lot? The task list to set up this process correctly is endless and can overwhelm you anytime.
That’s where we can help you!
Reach out to us if you need help figuring all this out. We can help you strategise the compliance activities, plan the validation study, connect with labs, and manage the necessary documentation.
Drop an inquiry at contact@alceonconsulting.com or book a FREE consultation call from HERE.

